Research Summary
Questions Central to Our Research
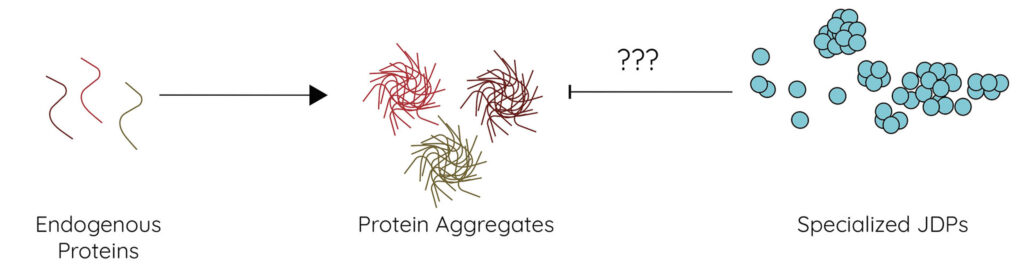
Question 1: How do specialized J Domain Protein (JDP) chaperones suppress protein aggregation?
To answer Question 1, our lab employs a broad array of biochemical and biophysical methods to better understand the relationship between JDP structural features, chemical features, and aggregation suppression activities in vitro and in cells.
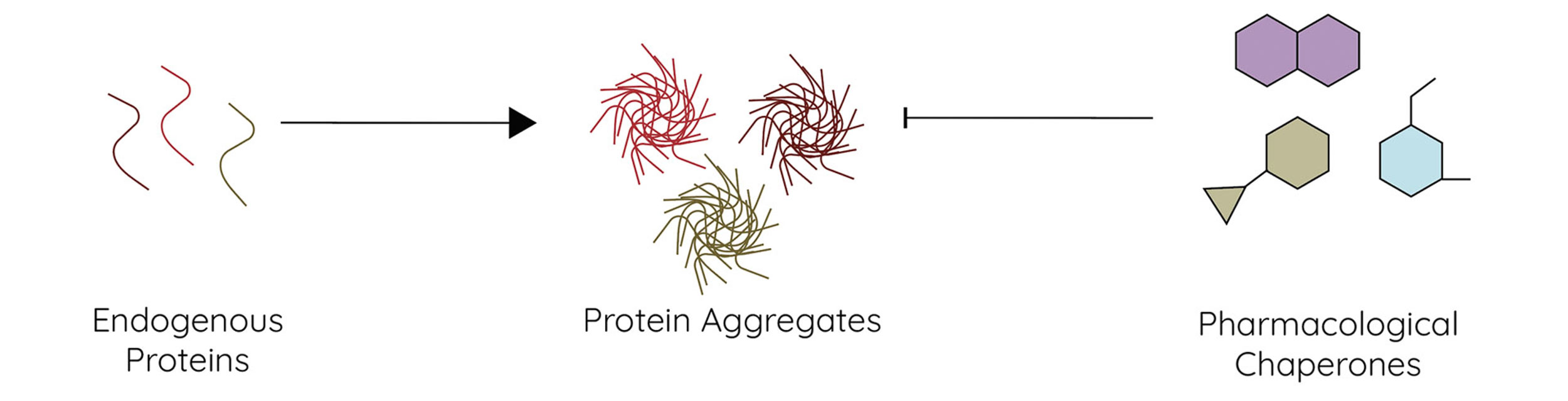
Question 2: How can we discover small molecules that prevent protein aggregation in disease?
To address Question 2, our lab uses biochemical and biophysical screening methods to identify small molecules that act as pharmacological chaperones, suppressing protein aggregation in protein misfolding diseases. Additionally, we anticipate that understanding the mechanistic underpinnings of JDP aggregation suppression will enable rational design of pharmacological chaperones.
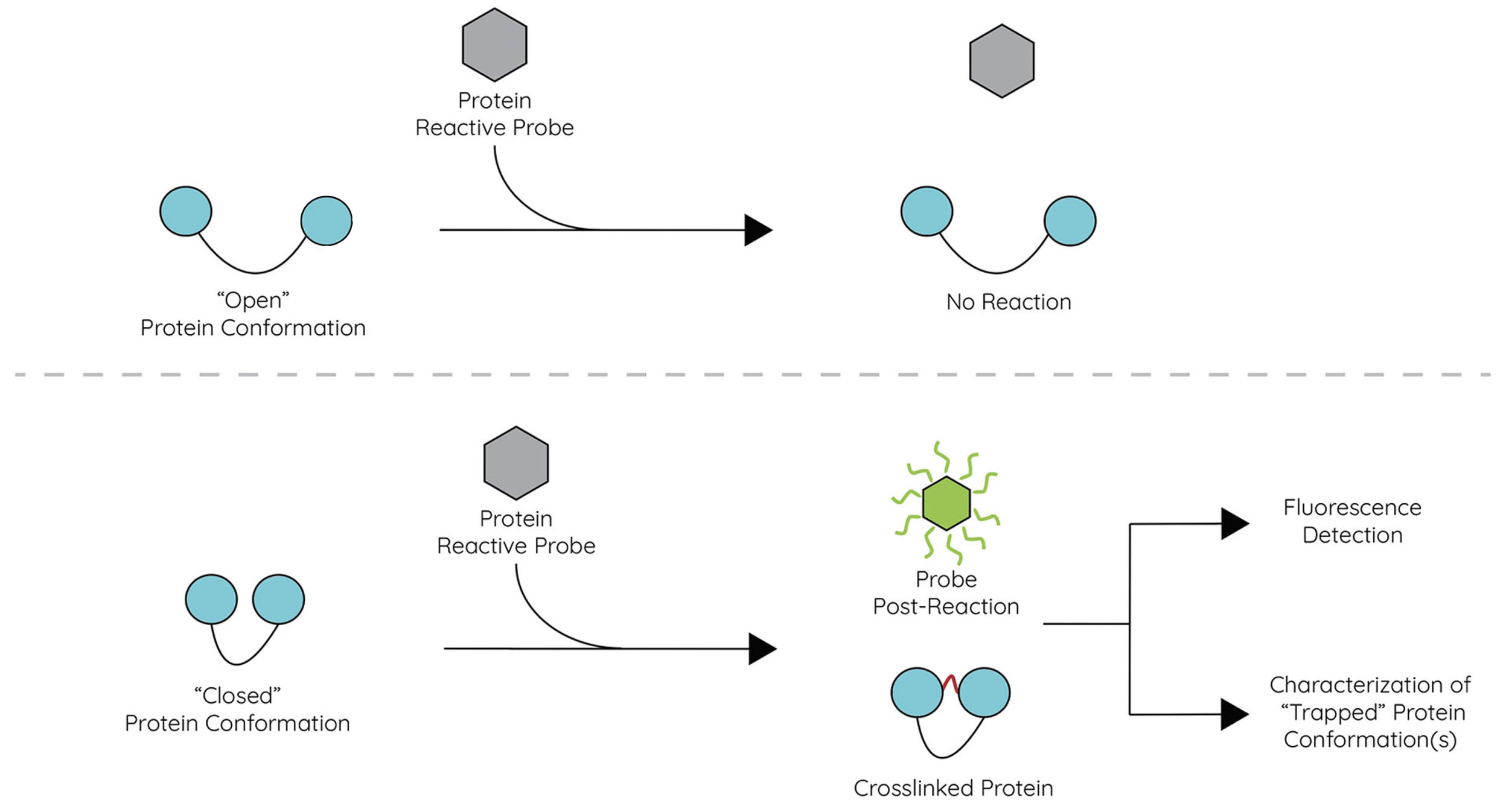
Question 3: How can we detect and report altered protein dynamics?
We are addressing Question 3 by developing assays and chemical probes to monitor the conformation of proteins in vitro and in cells. These tools will be used to screen for small molecules that alter or “trap” specific protein conformations, enabling high-throughput screening and discovery of pharmacological chaperones in an activity-agnostic manner.